Nearly a Billion in Hospital Community Benefits

INSIDE THE ISSUE
> Community Benefits
> 799 Patients Awaiting Transfer
> Prescribing Controlled Meds
> Digital Advisory Committee
MONDAY REPORT
Massachusetts Hospitals’ Community Benefits Increased in 2022
Massachusetts hospitals provided $904 million in community benefits to the cities and towns they serve in Fiscal Year 2022, which represented a marked increase over the previous year’s totals, according to the Massachusetts Attorney General’s Office.
Hospitals detail their community outreach efforts in annual reports to AG in addition to submitting information to the Internal Revenue Service as required by the Affordable Care Act.
The total from FY22 (October 1, 2021, to September 30, 2022), which are the latest available in Massachusetts, show that 48 non-profit hospitals reported contributing $840 million in total community benefits expenditures (up from $767 million in FY21), and nine investor-owned hospitals reported $64 million in community benefits expenditures (up from $30 million in FY21). Five HMOs provided $144 million in community benefits.
(The IRS allows hospitals to count financial losses related to care provided to Medicaid recipients, along with medical education costs and other metrics. In FY21, the IRS totaled Massachusetts hospital community benefits at more than $3.5 billion.)
“This year’s reports underscore the continued investment of our healthcare system to foster healthy communities across the commonwealth,” said Attorney General Andrea Campbell. “Through these community benefits, our hospitals and HMOs strengthen diverse community programs to address community health needs, particularly in the areas of mental health and substance use disorder.”
The hospitals’ support for their communities came during one of the most financially destabilizing fiscal years in recent memory. According to the Center for Health Information and Analysis’ Hospital & Health System Financial Performance report for FY 2022, the statewide median acute hospital operating margin was -1.3%, a decrease of 2.1 percentage points compared to FY2021. Acute hospitals reported $1.5 billion in temporary staffing costs in FY 2022, more than double the amount reported in the prior fiscal year.
A hospital’s essential community benefits programs generate real-world results for entire populations and help bridge health equity disparities. As they help lift one section of society, their positive results reverberate throughout entire communities and affect generations. For example, hospital-based outreach to improve housing quality can reduce asthma rates in children, which improves school scores, which lowers hospital admissions and healthcare costs – and on and on.
The FY22 programs were developed under the AG Office’s revamped guidelines that stress hospital and HMO efforts to address health equity while promoting investments in social determinants of health.
To see the hundreds of programs that were offered throughout the state in FY22 – many of which are continuing today – visit the AG’s Community Benefits website.
799 Patients in Acute Care Hospitals Are Awaiting Discharge
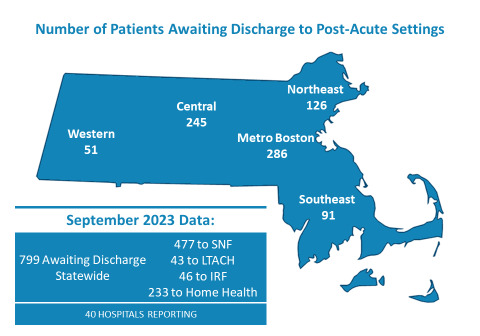
MHA’s most recent Throughput Survey Report from September 2023 shows there were 799 patients awaiting discharge from both acute care and post-acute care hospitals. But due to a variety of reasons – from staffing shortages at post-acute facilities, to insurer administrative burdens, or the lack of guardianship, conservatorship, or healthcare proxies, among other factors – patients that are ready to be discharged cannot be moved.
When patients cannot be transferred, they occupy beds that are needed by other patients that are backed up in emergency departments awaiting an inpatient bed.
The latest data shows that of the 799 patients in September, 477 were awaiting transfer to a skilled nursing facility (SNF). Forty-three percent of patients awaiting to be discharged to a SNF were waiting for 30 days or more. Between August and September, patients awaiting discharge to a SNF increased by 12%.
Telemedicine Flexibilities Extended for Controlled Substance Prescriptions
Medical practitioners will be able to prescribe controlled medications through December 31, 2024, to patients they have only interacted with through telemedicine, due to a temporary rule the Drug Enforcement Administration (DEA) issued earlier this month.
Before the pandemic Public Health Emergency, a patient in most instances would have to establish an in-person relationship with a practitioner before buprenorphine or certain controlled substances could be prescribed. That requirement was waived during the pandemic and the DEA extended that waiver in May 2023 to November 11, 2023.
But this month, the DEA, citing 38,000 written comments it received (including those from MHA and the Massachusetts Telemedicine Coalition or tMED), as well as additional comments gathered in listening sessions, said it needed more time to thoughtfully craft a final rule. The extension of the telehealth flexibility to the end of next year will allow another comment period to occur, the DEA said.
Want to Serve on an FDA Digital Health Advisory Committee?
The digital component of healthcare – artificial intelligence, virtual reality, cybersecurity, health data transfer, among many other topics – is advancing exponentially. To address the rapidly changing landscape the U.S. Food & Drug Administration (FDA) is creating a nine-member Digital Health Advisory Committee and is accepting nominations for it through December 11, 2023.
According to the FDA: “The Digital Health Advisory Committee will advise the Commissioner of Food and Drugs on issues related to Digital Health Technologies (DHTs), and FDA policies and regulations about these technologies, providing relevant expertise and perspective to improve the FDA’s understanding of the benefits, risks, and clinical outcomes associated with use of DHTs, as well as identifying risks, barriers, or unintended consequences that could result from proposed or established FDA policy or regulation for topics related to DHTs. This also may include advice on the use of DHTs in clinical trials or post-market studies subject to the FDA’s regulation.
The FDA Commissioner will choose committee members. Learn more here, including how to apply.

 Massachusetts Health & Hospital Association
Massachusetts Health & Hospital Association